New Treatment System Set to Launch Amid Hopes and Regulatory Challenges
Advanced 사설 카지노 medicine treatments, previously unavailable in South Korea, will be accessible starting February 21, marking a potential breakthrough for patients with rare and intractable diseases. While expectations are high that this change will improve accessibility and reduce the need for overseas treatment, concerns persist regarding the safety of high-risk procedures and the financial burden on patients.
On February 19, the 사설 카지노 Medicine Promotion Foundation, in collaboration with government agencies, hosted a briefing at the Korea Press Center. The event, held in preparation for the revised implementation of the Advanced 사설 카지노 Medicine and Advanced Biopharmaceuticals Safety and Support Act (hereafter "Advanced 사설 카지노 Bio Act"), attracted over 300 attendees, including representatives from medical institutions and cell processing facilities.

So-ra Park, President of the 사설 카지노 Medicine Promotion Foundation, emphasized, "Although South Korea enacted the Advanced 사설 카지노 Bio Act in 2019 to promote clinical research, the lack of a treatment system forced many patients to seek care abroad. Establishing evaluation criteria for treatment plans and managing patient costs remain challenges, but collaboration among government, industry, and medical institutions is essential to ensure transparency and reduce financial burdens."
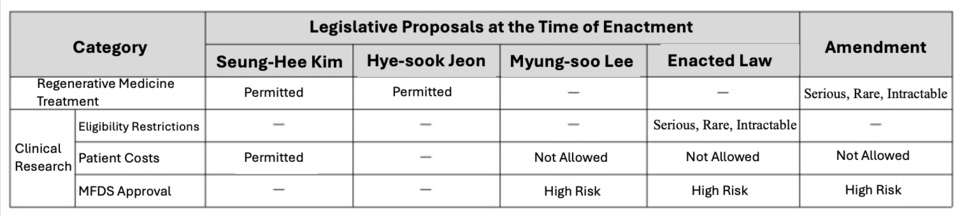
The Advanced 사설 카지노 Bio Act, implemented in 2020, initially permitted only clinical research rather than treatment, restricting direct patient access. The revised law expands clinical research to all diseases beyond serious, rare, and intractable conditions while strengthening safety management requirements.
Under the new system, 사설 카지노 medicine treatments classified as high- or moderate-risk can only be provided by designated institutions approved by the Ministry of Health and Welfare (MOHW) after completing preclinical research and treatment plan reviews. Low-risk treatments, however, may proceed without preclinical research.

Public-Private Collaboration for Safe, Affordable, and Accessible Treatments Needed

Soon-gil Jeong, Director of the 사설 카지노 Medicine Policy Division at MOHW, noted, "Until now, only a small, pre-selected group of patients could access advanced 사설 카지노 medicine treatments as part of research. With the new system, more patients will benefit from these medical advancements."
Beyond patient access, he highlighted that the system aims to foster technology development and commercialization. 사설 카지노 medicine faces challenges such as limited animal testing models and small patient recruitment pools for clinical trials. However, by systematically collecting clinical evidence and demonstrating safety and efficacy, 사설 카지노 medicine can transition from an experimental phase to a mainstream medical practice.
The Advanced 사설 카지노 Bio Act integrates four legislative proposals. In February last year, the National Assembly passed a bill removing clinical research restrictions and establishing a 사설 카지노 medicine treatment framework. The government aims to accelerate innovation in this field, with the first treatments under the revised act launching on February 21.
Jae-min Kim, an official from the 사설 카지노 Medicine Policy Division, pointed out that "Korea lags behind global standards in investigator-initiated clinical trials and is overly focused on cell therapy. Unlike the U.S. and Japan, which improved accessibility through legal reforms, Korea has not approved any new 사설 카지노 treatments since 2020. This lack of domestic treatment options has led to increased overseas medical trips and unregulated procedures."
However, he added, "The government is prioritizing 사설 카지노 medicine as a strategic national technology. As the participant pool in clinical research has expanded over the past five years, the scope of 사설 카지노 medicine treatments may also grow in the future, depending on their effectiveness."
With the revised law in effect, regulations on 사설 카지노 medicine institutions will be reinforced. Designated institutions must report detailed treatment data, including patient eligibility, administration records, costs, adverse reactions, and outcomes, to the National Institute of Health, which oversees safety management. Institutions will also undergo periodic inspections and on-site evaluations.
Since advanced 사설 카지노 medicine treatments are not covered by national health insurance, patients bear the full cost of these high-priced procedures. To prevent fraudulent billing, the government will closely monitor medical institutions.
Stricter Oversight and Penalties for Violations

Min-jae Kim, another official from the 사설 카지노 Medicine Policy Division, stated, "We are working to add advanced 사설 카지노 medicine treatments to the list of non-reimbursable services under National Health Insurance reimbursement rules. The Advanced 사설 카지노 Medicine Review Committee will establish cost guidelines, and institutions that overcharge or submit fraudulent reimbursement claims may face legal action."
Seung-hyun Kim, a researcher from the 사설 카지노 Medicine Safety Management Division at the National Institute of Health, emphasized, "Designated institutions must submit regular treatment reports through the safety management system. We plan to conduct annual site inspections and field audits to ensure compliance."
For the first time, the briefing introduced approval criteria and compliance requirements for cell processing facilities involved in 사설 카지노 medicine, attracting significant attention. According to the Ministry of Food and Drug Safety (MFDS), as of the end of last year, 50 cell processing facilities had been approved in key regions, including Seoul, Gyeongin, Busan, Gwangju, and Daejeon.
Nam Joo-seon, a researcher from the Advanced Biopharmaceuticals Task Force at MFDS, explained, "Facilities handling human cells, tissues, and organs for 사설 카지노 medicine must meet stringent requirements to receive approval. They must also renew their licenses at least 120 days before expiration every three years."
Meanwhile, under the Advanced 사설 카지노 Bio Act, 112 medical institutions had been designated as advanced 사설 카지노 medicine providers by the end of last year. The designation process under the revised law will begin in April.
Hyung-jun Kim, head of the Institution Designation Team at the 사설 카지노 Medicine Promotion Foundation, announced, "Applications for 2025 designation will open between April and May. Interested institutions should thoroughly review the updated legal requirements and submit necessary documents accordingly."
