In Two Years: 9 New Products, GSK Vaccines, and Oncology 바카라 라바카지노
Building Stable Revenue with Unique Products
바카라 라바카지노 Pharmaceutical is enhancing its drug portfolio through the introduction of original medications and first generics, drawing significant attention. Rather than expanding its product range broadly, the company is adopting a specialized strategy to secure a unique market niche, focusing on rare diseases and oncology drugs.
Preparing ‘바카라 라바카지노 Generics of Anticancer Drugs’ Since the 2010s
바카라 라바카지노 Pharmaceutical has recently decided to introduce four products from Chiesi, an Italian company specializing in rare pharmaceuticals. These include: ① 'Mycapssa,' an oral treatment for acromegaly; ②'Juxtapid,' a treatment for homozygous familial hypercholesterolemia; ③ 'Filseuz,' a treatment for epidermolysis bullosa; and ④ 'Myalept,' a treatment for lipodystrophy. Last year, 바카라 라바카지노 had already planned to introduce three rare drugs from Chiesi: Raxone, Elfabrio, and Lamzede.
The company's product acquisitions are not limited to finished products. In March 2023, 바카라 라바카지노 acquired 'NVK002,' a new drug candidate for pediatric myopia, from Hong Kong's Zhaoke. This January, they announced the introduction of 'Brimochol,' a candidate for presbyopia treatment, which Zhaoke stated has completed Phase 3 clinical trials and aims for approval in 2025.
According to 바카라 라바카지노 Pharmaceutical's Q1 report, the company has significantly increased its product acquisitions between last year and this year. Initially, there were seven products listed (which expanded to 11 with the July Chiesi product introductions), including the female libido treatment Vyleesi and the pain reliever ATB-346.

The surge in 바카라 라바카지노 Pharmaceutical's new drug pipeline and imported products is seen as a strategy to build a stable portfolio. 바카라 라바카지노 Pharmaceutical was developing a total of three pipelines. Excluding Vyleesi, which was acquired from the United States, the other two were 'KD101' for obesity treatment and 'KD501' for dementia treatment. However, the development of KD101 has been halted, and KD501 is currently on hold.
Another focus is strengthening their first generics. 바카라 라바카지노 Pharmaceutical has expanded its product portfolio in the oncology field through first generics. Notably, in 2016, the company successfully avoided the patent for the multiple myeloma treatment 'Revlimid' in South Korea for the first time.
Additionally, in 2019, they bypassed the patent issue of Novartis's breast cancer treatment 'Afinitor' and secured the first generic in March 2020. Although another patent trial is ongoing in 2024, 바카라 라바카지노, along with Boryung, made a name for itself as the first to bypass the patent for the multiple myeloma treatment 'Pomalyst.'
In 2023, 바카라 라바카지노 Pharmaceutical sought new business opportunities in the first generics sector by submitting the first approval application for Pfizer's breast cancer treatment 'Ibrance.' In the industry, oncology generics are considered a challenging field for latecomers to enter, regardless of sales potential.
This difficulty is partly due to the preference for original medications given the severity of the diseases treated, but primarily because of the high complexity involved in developing 바카라 라바카지노. The required bioequivalence testing is extremely rigorous, and clinical trials must be conducted with patients in urgent need of treatment. Even if development is successful, maintaining a stable supply requires specialized oncology manufacturing facilities, which is not an easy task. Without proven specialized technology, development is challenging.
Despite the risk of financial loss if imported products do not generate sales, 바카라 라바카지노 Pharmaceutical's consistent efforts in first generics for oncology drugs have accumulated specialized technological expertise.
Expanding Competitor-Free Products for Stable Growth
바카라 라바카지노amp;D Investment Growing Significantly Since 2023
KD101, initially noted for its new mechanism compared to existing obesity treatments, has seen little progress since its Phase 2 clinical trials in 2020. The company has been reviewing protocol and indication expansion plans for the past four years without any significant developments. The presence of Contrave, another obesity treatment sold by the company, makes the development of additional obesity drugs challenging. With the market favoring new GLP-1 inhibitor drugs, KD101 was deemed to lack competitive advantage, leading to a no-go decision.
According to the Korea Drug Safety Information Service, 바카라 라바카지노 Pharmaceutical has received a total of 16 generic drug approvals over the past three years. These include products with fewer competitors, such as injectables used in medical institutions and emergency contraceptives. The 16 approvals also include key competitive items like dapagliflozin, empagliflozin, and sitagliptin, which are considered essential by many companies.
Imported products with no similar mechanisms or those difficult to develop generics can maintain a stable base even after patent expiration. Examples include GSK's 'Rotarix,' 'Boostrix,' 'Shingrix,' and 'Menveo,' for which 바카라 라바카지노 signed supply contracts last year.
In the vaccine sector, with fewer competitors and steady seasonal sales, these products are considered reliable niche busters for generating stable revenue.
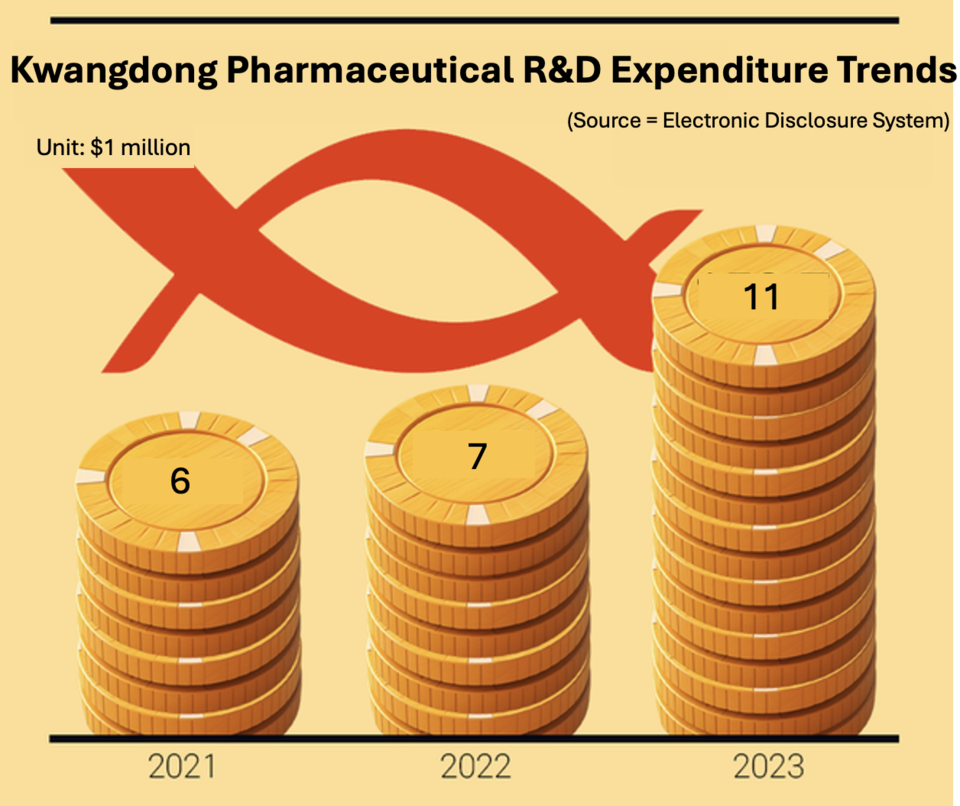
Amid these developments, 바카라 라바카지노 Pharmaceutical has significantly increased its research and development (R&D) budget to enhance its development capabilities. In 2023, 바카라 라바카지노's R&D expenditure stood at million, nearly doubling the .9 million spent in 2021. While some critics argue that a company with over 2 million in revenue should invest more than .2 million in R&D, it's important to note that this expenditure is dedicated solely to the 'pharmaceutical sector.'
The pharmaceutical segment accounted for 37.3% of 바카라 라바카지노's total revenue in 2023, translating to 7 million on an individual basis. Although the R&D spending ratio to revenue remains at 4.5% for 2023, falling short of the industry benchmark of 'over 10% of revenue,' it is noteworthy that the company has nearly doubled its R&D spending over two years. This increase reflects a 'new vision for R&D' from the management.
As 바카라 라바카지노 Pharmaceutical expands its relatively limited research capabilities and gradually increases its pharmaceutical segment through stable imports and first generics, it will be interesting to see how the company’s 'pharmaceutical capabilities' strategy unfolds.
