Professor In-Ho Kim presents clinical significance of 사설 카지노 as a game-changer in Urothelial carcinoma therapy at KSMO conference.

Astellas Pharma's groundbreaking drug, 사설 카지노 (active ingredient: Enfortumab vedotin/EV), is poised to redefine the treatment landscape for urothelial carcinoma. Professor In-Ho Kim, from the Oncology Department at Catholic University of Korea Seoul St. Mary's Hospital, presented the clinical significance of 사설 카지노, the first antibody-drug conjugate (ADC) approved for both localized (LA) and metastatic (M) urothelial carcinoma (UC) during the Korean Society of Medical Oncology (KSMO) conference held at the Grand Walkerhill Seoul Hotel on September 7th.
Professor In-Ho Kim highlighted the increasing prevalence of bladder cancer in South Korea, affecting approximately 5,000 new cases each year, particularly among men, smokers, and the elderly population.
Currently, first-line treatments primarily include platinum-based chemotherapy and the immune checkpoint inhibitor "Keytruda (active ingredient: Pembrolizumab)," which is approved but awaits reimbursement.
Professor In-Ho Kim explained that 사설 카지노 is an ADC therapy targeting the cell surface protein Nectin-4, expressed in UC. Importantly, 사설 카지노 remains effective irrespective of Nectin-4 expression, eliminating the need for pre-biomarker testing.
Nectin-4 is characterized by low expression in healthy cells but significantly higher expression in UC, present in approximately 80% of UC cells and around 60% of bladder cancer cases.
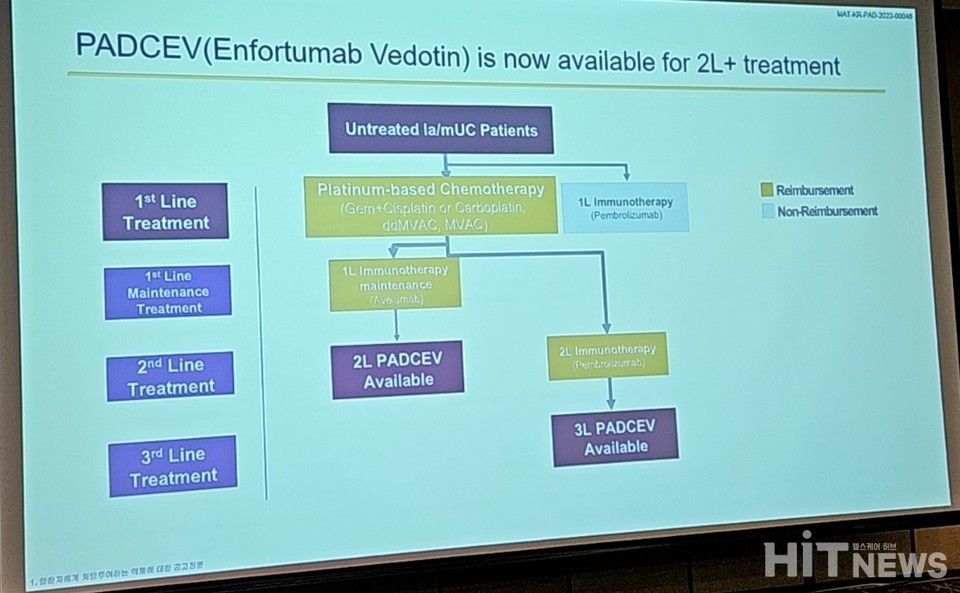
Professor Kim emphasized that 사설 카지노's approval has transformed the landscape of second-line treatments, replacing options like chemotherapy and immunotherapy.
사설 카지노 gained approval in March as a second and third-line treatment for "locally advanced or metastatic urothelial carcinoma patients who did not respond to platinum-based chemotherapy and anti-PD-L1 immune checkpoint inhibitors." Notably, the pivotal clinical study 'EV-301' demonstrated that Enfortumab vedotin (EV) significantly improved key clinical outcomes compared to standard chemotherapy.
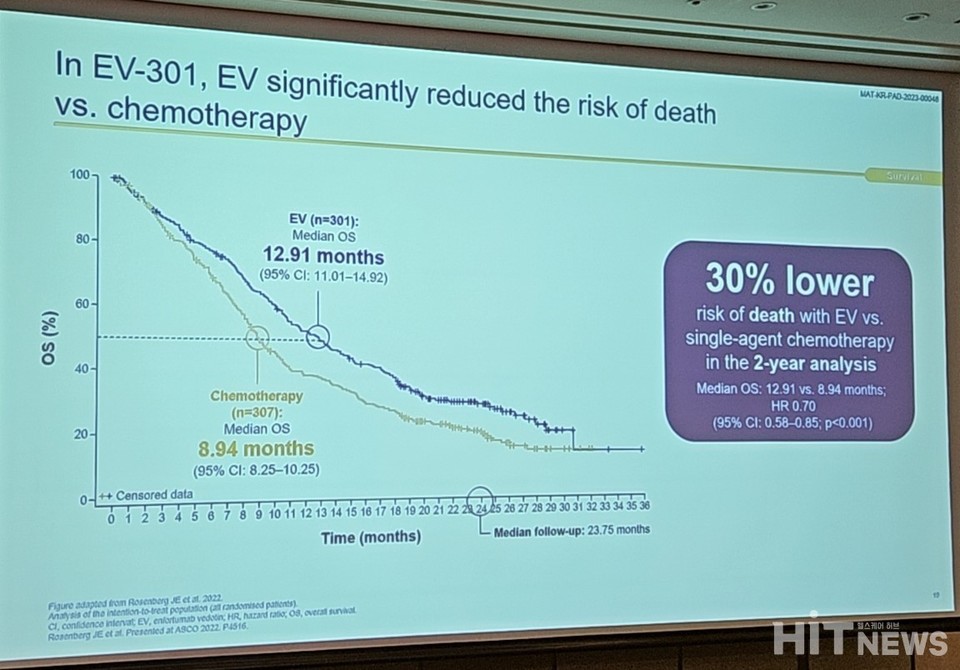
According to Professor Kim, the central tracking observation analysis of the EV-301 study at 23.75 months revealed a median overall survival (OS) of 12.91 months. This represented a 30% reduction in the risk of death compared to the control group (HR=0.704, 95% CI: 0.581-0.852, P=0.00015).
Furthermore, the median progression-free survival (PFS) improved to 5.55 months compared to 3.71 months in the control group, with a 37% lower risk of disease progression and death (HR=0.632, 95% CI 0.525-0.762, P<0.00001).
In terms of safety, EV-301 did not report any notably severe adverse events (AEs). Common adverse reactions included skin issues, hyperglycemia, peripheral neuropathy, and ocular disorders, primarily at grades 3 or lower. These events typically occurred within the first few months of treatment and were manageable with dose adjustments.
Furthermore, global guidelines now strongly recommend EV for patients with locally advanced and metastatic urothelial carcinoma who have previously received PD-1/L1 inhibitors and chemotherapy.
Professor Kim highlighted that both the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) are prioritizing 사설 카지노 based on the EV-301 study results. However, the drug's cost remains a barrier for many patients, and there is hope for future insurance coverage to make it accessible to a broader population
