EV-302/KEYNOTE-A39: 13% Greater Death Risk Reduction in 안전한 바카라 사이트s
Neurotoxicity, Hyperglycemia More Frequent
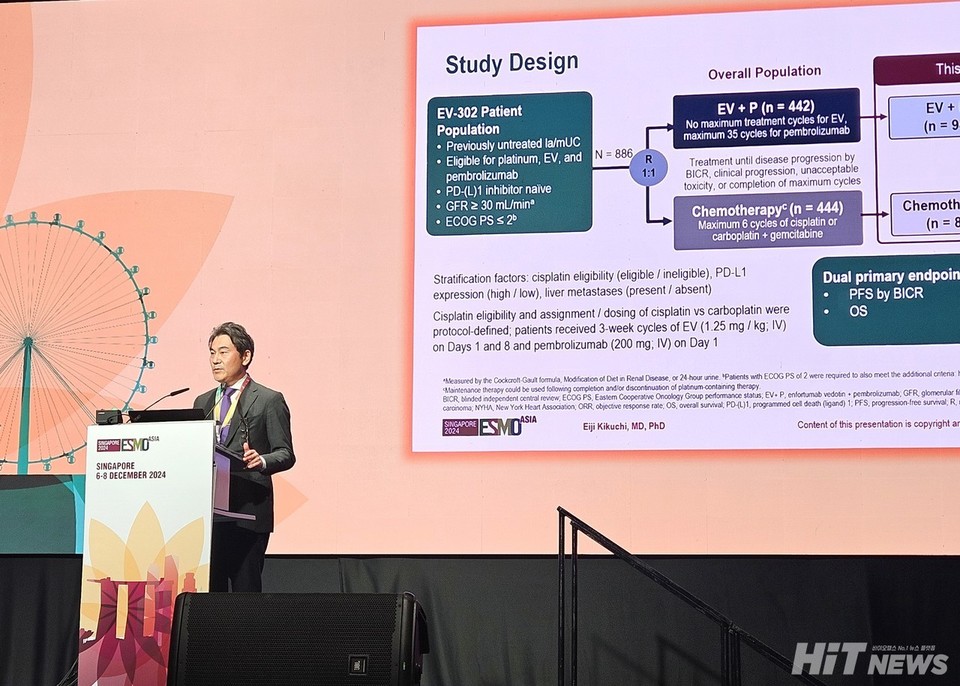
A recent sub-analysis of the Phase 3 EV-302/KEYNOTE-A39 clinical trial demonstrated that the combination therapy of Keytruda (pembrolizumab) and Padcev (enfortumab vedotin) significantly reduces the risk of death for 안전한 바카라 사이트 patients with urothelial carcinoma. Compared to chemotherapy, the combination therapy showed a 66% reduction in the risk of death in this population.
Presented at the ESMO Asia 2024 Congress on December 7, the sub-analysis included 176 안전한 바카라 사이트 patients from South Korea, China, Japan, Singapore, and Taiwan. These patients were part of the larger global trial, which enrolled 886 participants. Among the 안전한 바카라 사이트 cohort, 94 patients received the Keytruda-Padcev combination therapy, while 82 were treated with chemotherapy.
Professor Eiji Kikuchi of St. Marianna University School of Medicine in Japan noted that the combination therapy demonstrated significant improvements in progression-free survival (PFS) and overall survival (OS), key endpoints of the trial. The efficacy was more pronounced in 안전한 바카라 사이트 patients than in the global trial population.
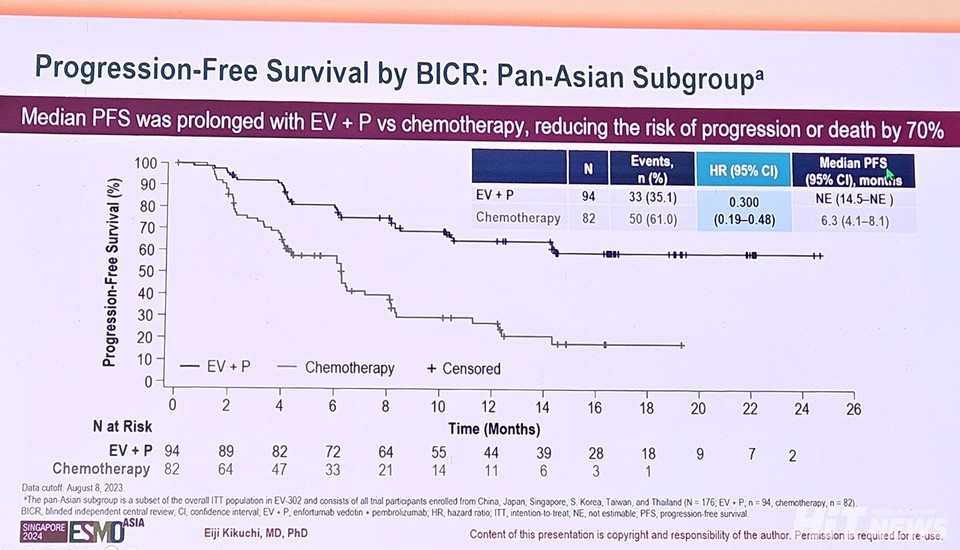
In the 안전한 바카라 사이트 cohort, the median PFS for the Keytruda-Padcev group has not yet been reached (95% CI: 14.5-NE), whereas the chemotherapy group recorded a median PFS of 6.3 months (95% CI: 4.1-8.1). This represents a 70% reduction in the risk of disease progression or death for the combination therapy group compared to chemotherapy, surpassing the 55% reduction observed in the overall population (HR: 0.45, 95% CI: 0.38-0.54, p<0.001).
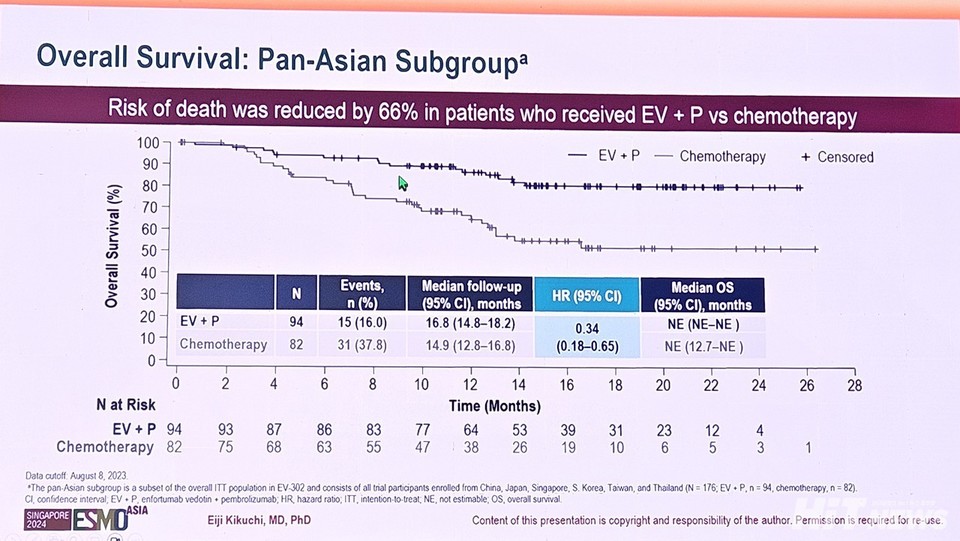
Similarly, the median OS in the 안전한 바카라 사이트 cohort was 16.8 months (95% CI: 14.8-18.2) for the Keytruda-Padcev group, compared to 14.9 months (95% CI: 12.8-16.8) in the chemotherapy group. This reflects a 66% reduction in the risk of death, which is an additional 13% improvement over the 53% reduction observed in the global patient population (HR: 0.47, 95% CI: 0.38-0.58, p<0.001).
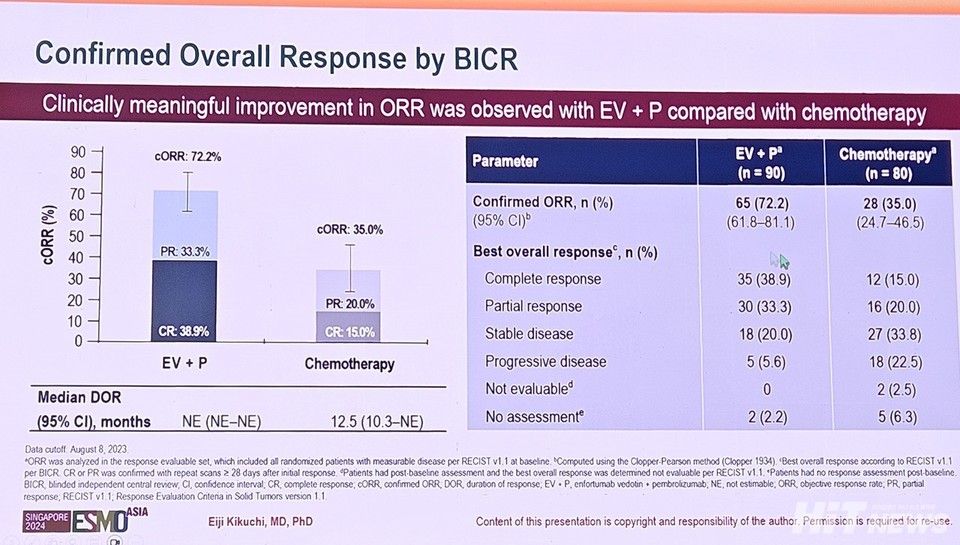
The objective response rate (ORR) was also notably higher among 안전한 바카라 사이트 patients. The Keytruda-Padcev group achieved an ORR of 72.2%, compared to 35.0% for chemotherapy, more than doubling the response rate. Globally, the ORR was 67.7% for the combination therapy and 44.4% for chemotherapy, highlighting the enhanced response in the 안전한 바카라 사이트 cohort.
Safety data confirmed that adverse events were manageable. Treatment-related adverse events (TRAEs) of grade 3 or higher occurred in 64.9% of the Keytruda-Padcev group and 68.4% of the chemotherapy group. However, 안전한 바카라 사이트 patients experienced slightly higher incidences of skin toxicity and hyperglycemia, consistent with the global safety profile.

Professor Kikuchi commented, “These findings support the use of Keytruda and Padcev as a new first-line treatment option for 안전한 바카라 사이트 patients with locally advanced or metastatic urothelial carcinoma. The safety profile was manageable, with no new safety signals observed.”
Padcev is already approved in South Korea for first-line use in combination with Keytruda for metastatic urothelial carcinoma and as a monotherapy for second-line or later treatment. Its developer, Astellas, recently submitted a reimbursement application for the combination therapy. However, the high cost of combining expensive anticancer drugs has been noted as a barrier to widespread clinical adoption. The robust results from this 안전한 바카라 사이트 sub-analysis may bolster the case for reimbursement approval, potentially increasing accessibility for patients in need.

