FLAURA2 바카라 게임 사이트 Sub-Analysis: Median PFS Extended by 6.1 Months with Combination Therapy

The Phase 3 FLAURA2 trial demonstrated that combining Tagrisso (osimertinib) with platinum-based chemotherapy significantly improved progression-free survival (PFS) compared to Tagrisso monotherapy in patients with EGFR-mutated non-small cell lung cancer (NSCLC) as a first-line treatment. The trial included patients with EGFR exon 19 deletion or exon 21 (L858R) substitution mutations in locally advanced or metastatic non-squamous NSCLC. Findings from the sub-analysis involving 169 바카라 게임 사이트 patients in the combination group and 164 in the monotherapy group were presented on December 7 at the ESMO Asia 2024 Annual Meeting.
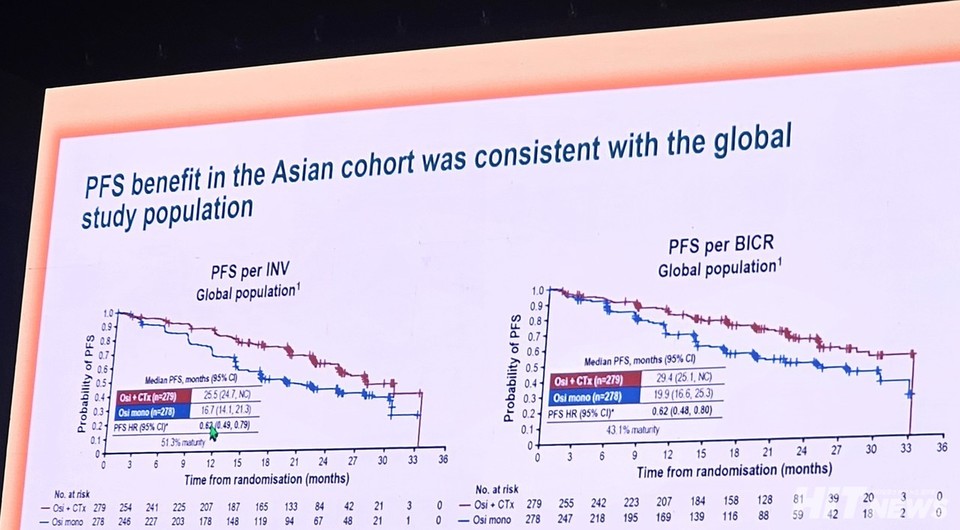
Professor James Chih-Hsin Yang from National Taiwan University Hospital shared the investigator-assessed median PFS results: 25.5 months (95% CI: 22.3–NC) for the combination therapy group, 6.1 months longer than the 19.4 months observed with monotherapy (HR 0.69, 95% CI: 0.51–0.94). Independent blinded central review (BICR) results further supported these findings, with the combination group showing a median PFS of 33.2 months (95% CI: 25.1–NC) compared to 19.9 months (95% CI: 19.2–NC) for monotherapy (HR 0.72, 95% CI: 0.52–1.01).
These results are consistent with the overall population analysis (ITT) presented at WCLC 2023, where combination therapy extended median PFS by 8.8 months and reduced the risk of disease progression or death by 38% (HR 0.62, 95% CI: 0.49–0.79, p<0.0001).
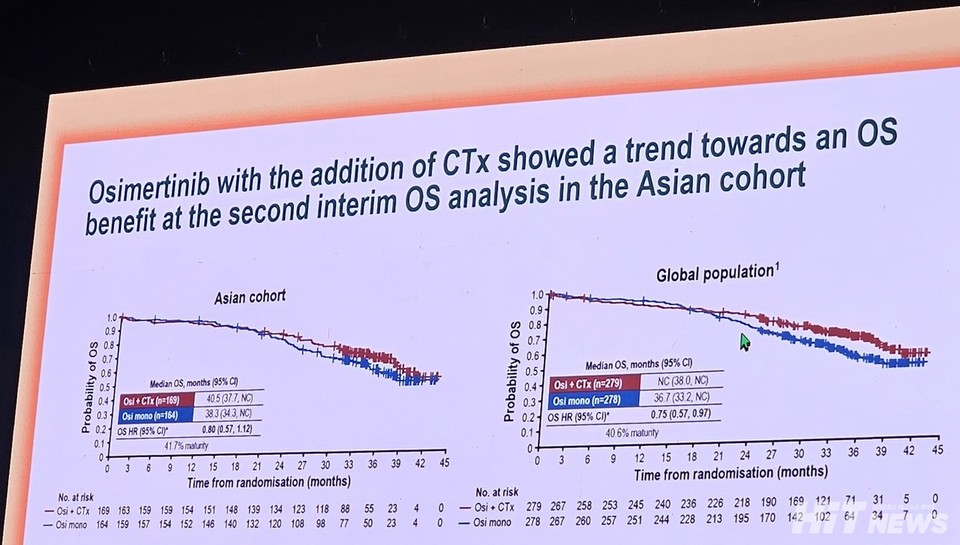
Median overall survival (OS) results for the combination group were 40.5 months (95% CI: 37.7–NC), compared to 38.3 months (95% CI: 34.3–NC) for monotherapy. However, the data are not yet mature, and statistical significance has not been achieved (HR 0.80, 95% CI: 0.57–1.12).
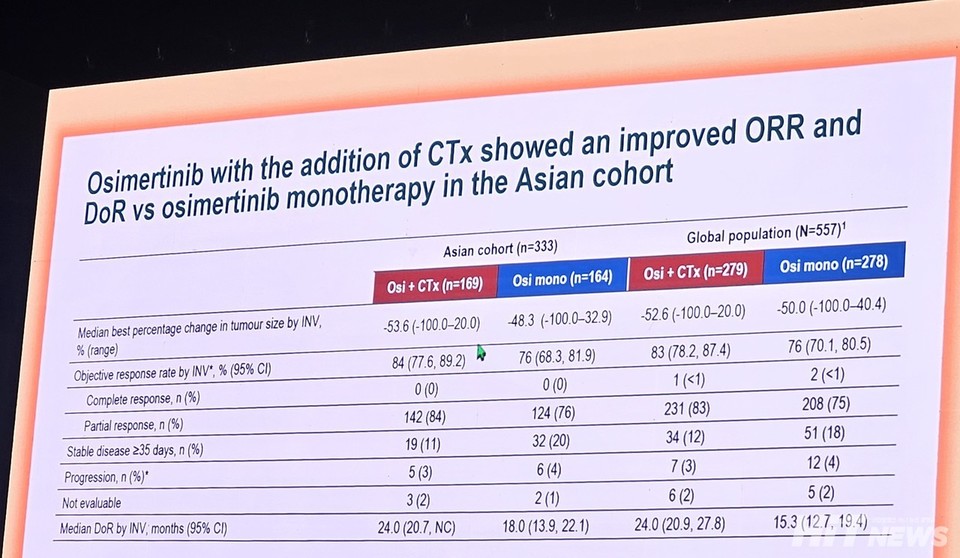
The 바카라 게임 사이트 cohort’s investigator-assessed objective response rate (ORR) was 84.0%, aligning with the global cohort (83.0%). Median duration of response (DoR) was also consistent at 24.0% (바카라 게임 사이트 cohort 95% CI: 20.7–NC; global cohort 95% CI: 20.9–27.8).
Adverse events of Grade 3 or higher were reported in 58% of the 바카라 게임 사이트 combination group, slightly higher than the global cohort (53%). The most common adverse events in the 바카라 게임 사이트 group were anemia (50%), diarrhea (38%), and nausea (37%).

Photo by Reporter Jaesun Hwang
Professor James Chih-Hsin Yang emphasized, “This sub-analysis confirmed a clinically meaningful PFS benefit and indicated a trend toward OS improvement as data continues to mature. These findings support the use of Tagrisso combined with chemotherapy as an additional first-line treatment option for 바카라 게임 사이트 patients with EGFR-mutated advanced NSCLC, beyond the existing monotherapy.”
AstraZeneca Korea obtained regulatory approval on April 15 for the combination of Tagrisso with platinum-based chemotherapy, making it the first EGFR tyrosine kinase inhibitor (TKI) in South Korea available for both monotherapy and combination therapy as a first-line treatment for EGFR-mutated non-squamous NSCLC.
관련바카라 게임 사이트
- 타그리소+화학요법, EGFR 폐암 1차 치료 아시아 환자 PFS 혜택
- "환자단체 첫 초청한 에볼루션 바카라… '환자 목소리' 중요성 인정받아 보람"
- 아태지역 무료 바카라 게임 단체, 싱가포르로 집결…'
- 수술 후 보조요법 허가 획득…적응증 넓혀가는 '타그리소'
- 타그리소-화학 병용, EGFR 변이 비편평 폐암 1차 치료제로 허가
- [WCLC23] "타그리소+화학항암 병용, 단독보다 mPFS 9개월 향상"
- "토토 사이트단체 첫 초청한 ESMO… '토토 사이트 목소리' 중요성 인정받아
- 타그리소 출시 전ㆍ후, 카지노 미국들의 치료 환경은 어떻게 개선됐나요?
- Leclaza + Rybrevant SC: Improved Outcomes in 바카라 게임 사이트 Patients
- ESMO Asia 2024: Uniting Patients and the Industry Against Cancer
- What do you th바카라 꽁머니k are the criteria for determ바카라 꽁머니바카라
- AZ, Daiichi’s TROP2 ADC Wins FDA Breakthrough Designation

