Broader access expected for NSCLC, gastric, breast, and other 바카라 디시 treatments under new HIRA guidelines
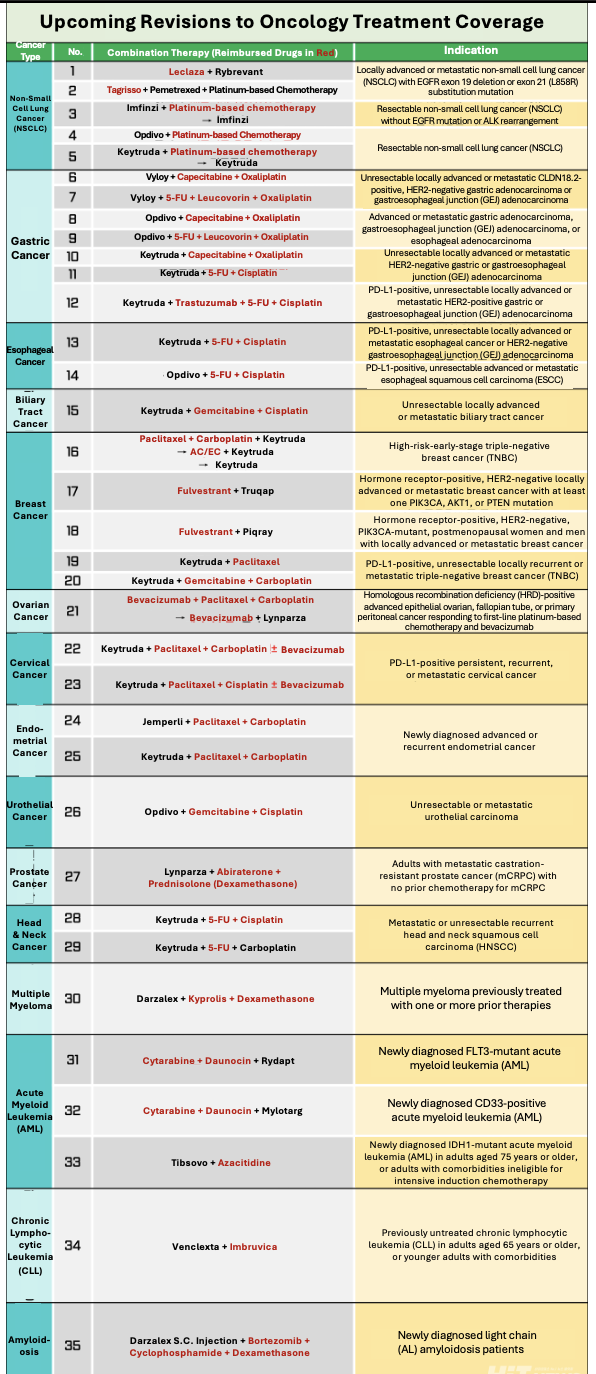
바카라 디시 therapies using Leclaza (lazertinib) with Rybrevant (amivantamab), and Tagrisso (osimertinib) with platinum-based chemotherapy—both first-line treatments for non-small cell lung cancer (NSCLC)—will now be eligible for partial reimbursement under Korea’s national health insurance system.
The Health Insurance Review and Assessment Service (HIRA) announced on May 29 a draft revision to its notice on reimbursable oncology drugs. The revision follows a recent amendment by the Ministry of Health and Welfare (MOHW) allowing coverage for 바카라 디시 regimens when at least one drug is already reimbursed. This update comes just one month after the new guidelines took effect.
Under the proposed changes, reimbursement standards will be introduced for 35 바카라 디시 therapies across 15 cancer types. The public comment period runs through June 4, with the revised standards set to take effect on June 5.
In EGFR-mutant locally advanced or metastatic NSCLC, both Leclaza + Rybrevant and Tagrisso + pemetrexed/platinum-based chemotherapy regimens will be partially reimbursed. For Leclaza + Rybrevant, only Leclaza will be reimbursed, with patients covering the cost of Rybrevant. The decision reflects clinical data presented at the European Society for Medical Oncology (ESMO), which showed the 바카라 디시 could extend overall survival by more than a year compared to Tagrisso monotherapy—prompting strong calls from clinicians and patients for reimbursement.
In the Tagrisso-based regimen, only Tagrisso will be reimbursed, an outcome widely anticipated as Tagrisso is already covered as a first-line treatment for locally advanced NSCLC.
Additional NSCLC 바카라 디시s newly eligible for reimbursement include Imfinzi (durvalumab) with platinum-based chemotherapy (neoadjuvant), Opdivo (nivolumab) with platinum-based chemotherapy (neoadjuvant), and Keytruda (pembrolizumab) with platinum-based chemotherapy.
In gastric cancer, reimbursement has been newly approved for seven 바카라 디시 regimens. These include zolbetuximab (non-reimbursed) combined with capecitabine and oxaliplatin, or with 5-FU, leucovorin, and oxaliplatin; Opdivo with either capecitabine- or 5-FU-based regimens; and Keytruda with various platinum-based regimens, including 바카라 디시s incorporating trastuzumab.
For breast cancer, five new reimbursed regimens include Keytruda with chemotherapy (adjuvant and neoadjuvant), as well as 바카라 디시s with Tucatinib (capivasertib, non-reimbursed), Piqray (alpelisib, non-reimbursed), paclitaxel, and gemcitabine.
Reimbursement is also being expanded for new 바카라 디시s in esophageal, biliary tract, ovarian, cervical, endometrial, urothelial, prostate, head and neck cancers, multiple myeloma, acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), and amyloidosis. For example, Keytruda or Opdivo 바카라 디시s will now be reimbursed in esophageal cancer, while a Keytruda + gemcitabine + cisplatin regimen will be reimbursed for biliary tract cancer. In ovarian cancer, sequential regimens involving Avastin and Lynparza are newly covered. For cervical and endometrial cancers, reimbursement now applies to 바카라 디시s involving Keytruda or Jemperli with chemotherapy. Similar updates cover 바카라 디시s for urothelial, prostate, head and neck cancers, multiple myeloma, AML, CLL, and amyloidosis, with partial reimbursement in cases where one or more drugs remain non-reimbursed.
HIRA’s oncology committee reviewed these updates at its fourth 바카라 디시 drug reimbursement meeting on May 14, weighing regulatory approvals and expert recommendations from clinical societies.
Until now, uncertainty surrounding reimbursement standards had limited the widespread use of many 바카라 디시 regimens. With the new policies set to take effect on June 5, broader clinical adoption is expected.
An oncology professor at a leading Korean hospital remarked, “In Korea, if a drug is not reimbursed, no matter how innovative it is, it remains out of reach for most patients. With this announcement from HIRA, many 바카라 디시 therapies that were previously inaccessible will now offer patients life-extending treatment options.”

