ASCENT-04/KEYNOTE-D19 Study Demonstrates Improved Progression-Free Survival in PD-L1-Positive Patients
A combination therapy of 슬롯 사이트 슬롯사이트 (pembrolizumab) and the TROP2 antibody-drug conjugate Trodelvy (sacituzumab govitecan) has shown promise as a new first-line standard treatment for patients with triple-negative breast cancer (TNBC).
On May 31 at the 2025 ASCO Annual Meeting, interim results from the Phase 3 ASCENT-04/KEYNOTE-D19 trial were presented. The study compared 슬롯 사이트 슬롯사이트 plus Trodelvy to 슬롯 사이트 슬롯사이트 plus chemotherapy in patients with PD-L1-positive, unresectable locally advanced or metastatic TNBC.
Dr. Sara M. Tolaney, Professor at Dana-Farber Cancer Institute and Harvard Medical School, noted: “Approximately 40% of patients with metastatic TNBC are PD-L1 positive. The current standard treatment is 슬롯 사이트 슬롯사이트 plus chemotherapy, but progression-free survival (PFS) is typically only 7.5 to 9.7 months. Most patients experience disease progression, and nearly half do not receive second-line therapy after progression.”
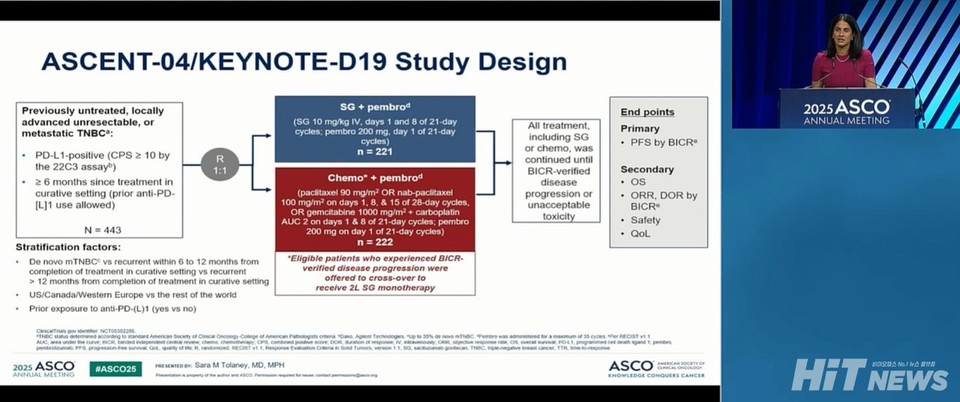
The ASCENT-04/KEYNOTE-D19 trial enrolled 443 patients with PD-L1-positive (CPS ≥10) unresectable locally advanced or metastatic TNBC. Participants were randomly assigned to receive either 슬롯 사이트 슬롯사이트 plus Trodelvy or 슬롯 사이트 슬롯사이트 plus chemotherapy (gemcitabine, carboplatin, paclitaxel, or nab-paclitaxel).
The primary endpoint was PFS, assessed by blinded independent central review (BICR). Secondary endpoints included overall survival (OS), objective response rate (ORR), duration of response (DOR), time to response (TTR), patient-reported outcomes, and safety.
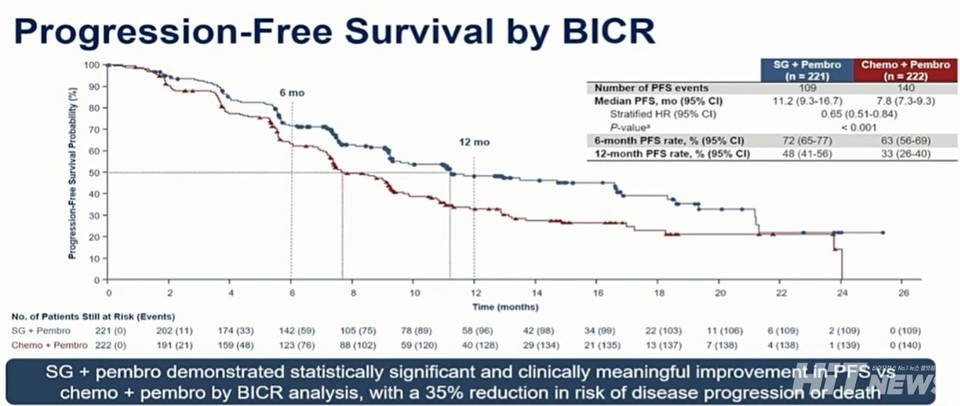
The 슬롯 사이트 슬롯사이트 plus Trodelvy group achieved a median PFS of 11.2 months (95% CI: 9.3–16.7), compared with 7.8 months (95% CI: 7.3–9.3) in the control group—a 35% reduction in the risk of disease progression or death (HR=0.65, 95% CI: 0.51–0.84, p<0.001). This benefit was consistent across all subgroups.
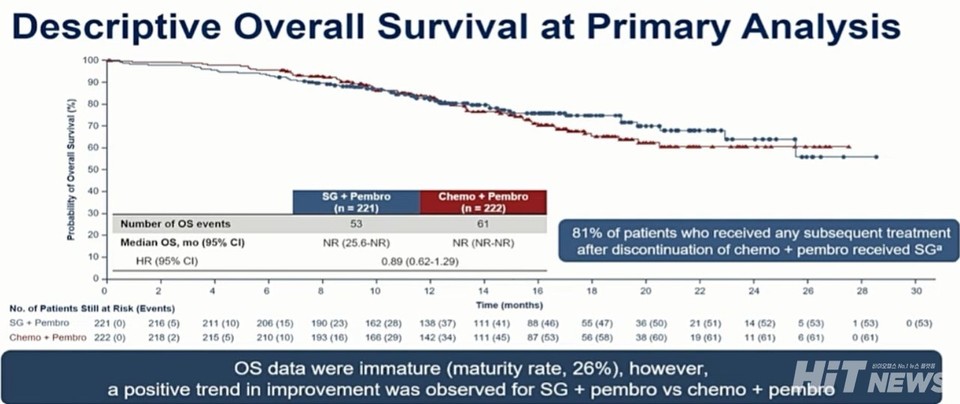
Median OS has not yet been reached (data maturity: 26%), though a positive trend was observed.
Professor Tolaney said, “The combination demonstrated a significant improvement in PFS and showed a positive trend in OS, supporting its potential as a new first-line standard therapy for 슬롯 사이트 슬롯사이트.”
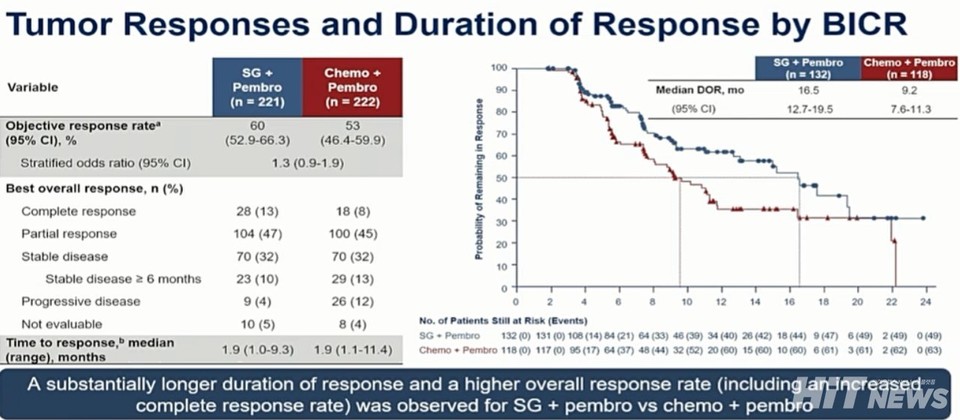
The ORR was 59.7% (95% CI: 52.9–66.3) in the combination group, with a complete response rate of 13%, compared to 53.2% (95% CI: 46.4–59.9) and 8% in the control group.
The DOR was also improved: 16.5 months (95% CI: 12.7–19.5) versus 9.2 months (95% CI: 7.6–11.3).
Grade 3 or higher adverse events occurred at similar rates (71% vs. 70%), but serious treatment-related adverse events were higher in the combination group (28% vs. 19%). Drug-related discontinuations were lower in the combination group (12% vs. 31%).
The most common Grade 3 or higher adverse events in the combination group were neutropenia (43%), diarrhea (10%), fatigue (8%), and anemia (7%).
Professor Tolaney emphasized that the safety profile was consistent with known side effects of each drug, with no new toxicities identified. She concluded: “These interim results highlight the potential of 슬롯 사이트 슬롯사이트 plus Trodelvy as a new standard first-line therapy for patients with significant unmet medical needs.”
Currently, Trodelvy is approved for TNBC patients who have received at least two prior therapies. Since 2021, MSD (Merck & Co.) and Gilead have collaborated to advance 슬롯 사이트 슬롯사이트 and Trodelvy into earlier lines of therapy.
The ASCENT-04/KEYNOTE-D19 trial, expected to conclude in May 2028, is being conducted at major hospitals in South Korea, including Seoul National University Hospital, Asan Medical Center, National Cancer Center, Samsung Medical Center, Korea University Anam Hospital, Yonsei Severance Hospital, Seoul St. Mary’s Hospital, and Bundang Seoul National University Hospital.

