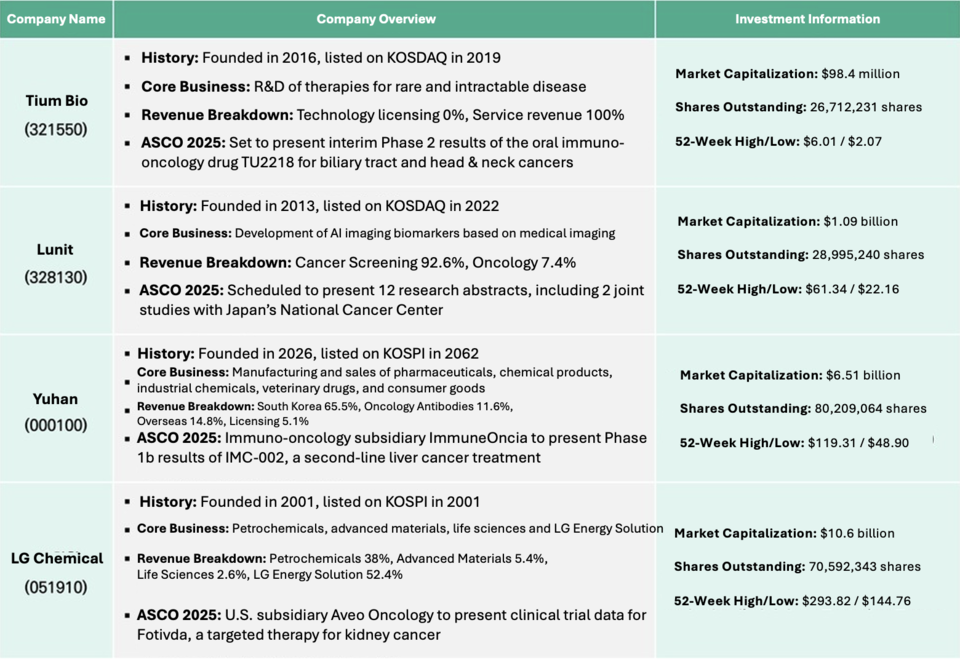
TiumBio, Lunit, ImmuneOncia, and Aveo unveil clinical innovations as the global oncology stage turns to AI, immunotherapy, and targeted treatments

The 2025 American Society of Clinical Oncology (바카라 카지노) Annual Meeting, scheduled from May 30 to June 3 in Chicago, will showcase the latest innovations in cancer treatment—from immunotherapy combinations to AI-driven biomarker technologies.
As one of the world’s top three oncology conferences, alongside AACR and ESMO, 바카라 카지노 draws over 40,000 global experts each year. A May 2 report by Growth Research named TiumBio, Lunit, and ImmuneOncia among the key Korean players to watch this year.
TiumBio Unveils Immuno-Oncology Drug ‘TU2218’
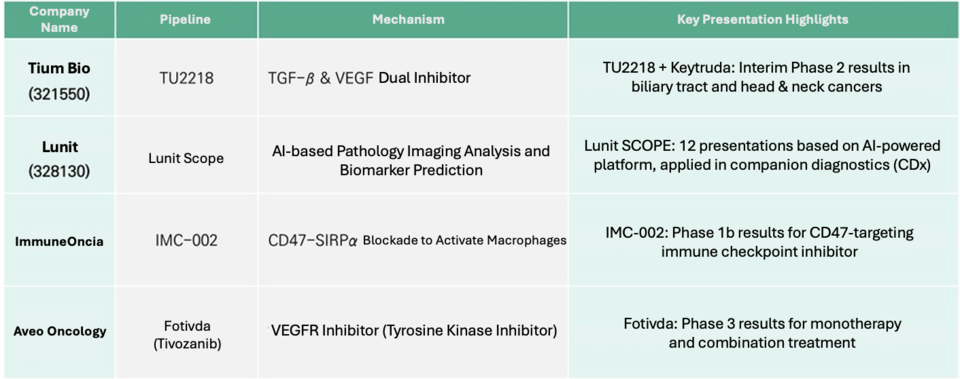
TiumBio will present interim Phase 2 results for TU2218, an oral immuno-oncology drug targeting head and neck as well as biliary tract cancers. The drug is designed to work in combination with Keytruda (pembrolizumab), aiming to boost response rates by inhibiting two critical cancer pathways simultaneously.
Currently, typical response rates in these cancers hover around 10–20%. TiumBio hopes to increase this to 30–40% with TU2218, potentially positioning the combination as a first- or second-line therapy for patients with few existing options.
Previous Phase 1b results presented at ESMO confirmed the safety of TU2218. With overall survival rates in these cancers typically ranging from 6 to 12 months, positive Phase 2a data could lead to future licensing opportunities.
Lunit ‘s AI-Based Biomarker Prediction Technology with 12 Presentations
Lunit is set to present 12 research abstracts at 바카라 카지노 2025, including two studies conducted in collaboration with Japan’s National Cancer Center East (NCCE). These studies focus on evaluating treatment response and tumor expression levels using Lunit’s AI-based biomarker platform, Lunit SCOPE.
The company aims to solidify Lunit SCOPE as a trusted AI biomarker in precision medicine and immunotherapy, emphasizing its potential as an essential tool in clinical decision-making. Lunit will also present research on quantifying target protein expression for antibody-drug conjugates (ADCs) using immunohistochemistry (IHC) imaging, further broadening its clinical applications.
As combination therapies involving immunotherapies and ADCs become more prevalent, Lunit anticipates mid- to long-term revenue growth through the use of Lunit SCOPE in companion diagnostics (CDx). Companion diagnostics are used to determine whether a patient is likely to respond to a specific treatment, thereby improving clinical outcomes while reducing costs.
In November 2024, Lunit signed a strategic agreement with global pharmaceutical company AstraZeneca. Since then, it has expanded its product pipeline, including AI tools to predict immunotherapy responses. The upcoming 바카라 카지노 presentation is expected to reinforce Lunit’s global presence in the oncology diagnostics market.
ImmuneOncia Presents Phase 1b Results for CD47 Antibody 'IMC-002'
ImmuneOncia, the immuno-oncology subsidiary of Yuhan Corporation, will present Phase 1b clinical results for IMC-002, a monoclonal antibody targeting the CD47 protein on cancer cells. As a macrophage immune checkpoint inhibitor, IMC-002 blocks the interaction between CD47 on tumor cells and SIRPα on macrophages, thereby enabling the immune system to recognize and attack cancer cells.
IMC-002 was previously licensed to China's 3D Medicines in 2021 in a deal valued at up to 0.5 million, including an upfront payment of million.
In addition to its current clinical program, ImmuneOncia is developing a new pipeline aimed at entering the bispecific antibody market. The company is also preparing for an upcoming KOSDAQ listing.
Aveo Highlights VEGFR Inhibitor for Kidney Cancer Treatment
Aveo Oncology, the U.S. oncology subsidiary of LG Chem, will present new clinical data on Fotivda (tivozanib), a targeted therapy for renal cell carcinoma, at the upcoming 바카라 카지노 2025 meeting.
Fotivda received regulatory approval from the European Medicines Agency (EMA) in 2017 and from the U.S. Food and Drug Administration (FDA) in 2021. The upcoming presentation will include findings on the drug’s safety and efficacy, featuring results from the Phase 3 TiNivo-2 trial, which evaluated both tivozanib monotherapy and its combination with the immune checkpoint inhibitor nivolumab.
According to Aveo, tivozanib monotherapy showed statistically significant improvement in progression-free survival (PFS), reinforcing its potential as a safe and effective treatment option for patients with advanced kidney cancer.
Which Drug Will Take This Year’s ‘Best of 바카라 카지노’?
At last year’s 바카라 카지노 meeting, the combination therapy of Rybrevant (amivantamab) by Johnson & Johnson and Leclaza (lazertinib) from Yuhan Corporation drew significant attention. Clinical results from a trial using a subcutaneous (SC) formulation of Rybrevant reported a 65% survival rate, a reduced administration time of five minutes, and an infusion-related reaction rate of just 13%.
Following the presentation, the Rybrevant–Leclaza combination received swift regulatory approvals: as a first-line treatment from the U.S. FDA in August and from the European Commission in December.
With recent precedents of 바카라 카지노 presentations leading to rapid global approvals, industry watchers are keen to see which new candidates will be named among this year’s “Best of 바카라 카지노.”

