With 1M Invested, 슬롯사이트 추천 Korea Expands Early Oncology Trials and Local Partnerships

To mark International Clinical Trials Day, 슬롯사이트 추천 Korea hosted the “R:IM” campaign event on May 19 in Seoul to showcase its ongoing R&D efforts and clinical trial achievements. At the event, Hyun-Joo Lee, Executive Director of Clinical Research, emphasized the critical role of Korean medical institutions in 슬롯사이트 추천’s global development strategy and underscored the company's continued investment in innovation.
Lee stated that 슬롯사이트 추천 Korea has invested over .7 million annually in R&D for the past four years, totaling 1 million since 2021. Last year alone, R&D spending reached .2 million—representing approximately 11% of 슬롯사이트 추천 Korea’s total sales. These investments have supported the introduction of 15 first-in-class drugs and vaccines in Korea, expanding the company’s portfolio to around 50 products, including immuno-oncology treatments, national immunization program vaccines, and novel antibiotics.

In 2023, 슬롯사이트 추천 Korea received the highest number of clinical trial approvals from the Ministry of Food and Drug Safety (MFDS), leading with 36 approvals. This outpaced AstraZeneca Korea (22), Addpharma (19), AbbVie Korea (17), and Boryung (15). From 2020 to 2024, 슬롯사이트 추천 Korea consistently received over 20 clinical trial approvals per year, with 48% being Phase 1 or 2 studies—highlighting its focus on early-stage development.
Currently, 슬롯사이트 추천 Korea is conducting 186 clinical trials across all therapeutic areas in collaboration with more than 640 domestic research institutions—161 of which are in oncology. Notably, 28% of 슬롯사이트 추천 Korea’s workforce, or 174 employees, are dedicated to R&D.
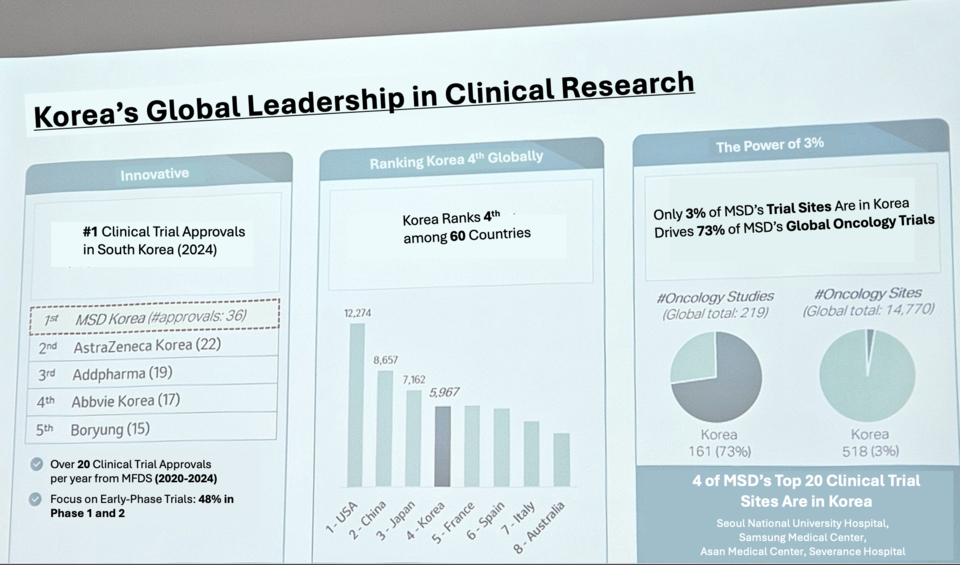
Amid evolving global clinical trial trends, Lee noted that countries participating in early-phase trials are increasingly continuing through to later stages, making early-phase involvement a key driver of access to innovative therapies. She stressed the importance of involving 슬롯사이트 추천n investigators from the outset of trial design, enabling them to contribute meaningfully to the global research landscape. 슬롯사이트 추천n clinicians are also gaining visibility as key speakers in Asia.
Korea now plays a strategic role in 슬롯사이트 추천’s global clinical operations, leading the Asia-Pacific region in the number of active clinical studies. Of the 14,770 global oncology trial sites 슬롯사이트 추천 operates, only 3% (518) are in Korea—but these account for 73% of the company’s global oncology clinical trial activity. Four of 슬롯사이트 추천’s top 20 clinical trial institutions worldwide are based in Korea: Seoul National University Hospital, Samsung Medical Center, Asan Medical Center, and Severance Hospital. With 5,967 enrolled patients, Korea ranks fourth globally in participation in 슬롯사이트 추천’s oncology trials, following the U.S. (12,274), China (8,657), and Japan (7,162).
In addition to clinical development, 슬롯사이트 추천 Korea continues to foster industry collaboration. The company is currently working with approximately 20 domestic pharmaceutical and biotech companies through technology transfers, co-promotion agreements, and joint research initiatives. It is also conducting 14 combination trials involving its immunotherapy Keytruda (pembrolizumab) with 11 local firms. New partnerships formed last year include collaborations with Samsung Biologics, Alteogen, ABL Bio, GI Innovation, and Hanmi Pharmaceutical.
Lee concluded, “Through robust partnerships and sustained investment, 슬롯사이트 추천 Korea is committed to advancing innovative medicines and vaccines in areas of unmet need. We will continue working closely with government agencies, academia, industry, and patient groups to help shape a healthier future for Korean patients.”

